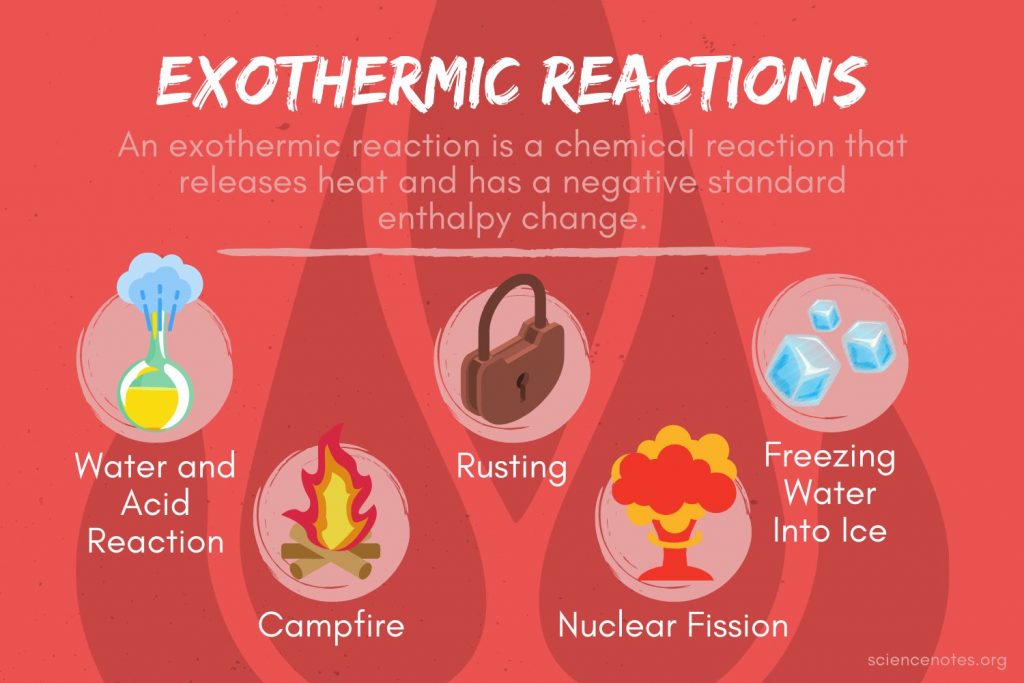
Heat Reaction Definition . Calorimetry is used to measure. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant.
from classnotes123.com
Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. Calorimetry is used to measure. The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.
What does one mean by exothermic and endothermic reactions? Give
Heat Reaction Definition When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in.
From www.slideserve.com
PPT Chapter 11 Thermochemistry Heat and Chemical Change PowerPoint Heat Reaction Definition The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. Calorimetry is used to measure. The heat of reaction which is also. Heat Reaction Definition.
From guides.co
States of Matter FD202 Fundamentals of Fire and Combustion on Guides Heat Reaction Definition One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. The heat of reaction (also known and enthalpy of reaction). Heat Reaction Definition.
From www.slideserve.com
PPT Unit 11 Thermochemistry PowerPoint Presentation, free download Heat Reaction Definition The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. Heat of reaction, the amount of heat that must be. Heat Reaction Definition.
From www.slideserve.com
PPT Heat of Reaction PowerPoint Presentation, free download ID6434217 Heat Reaction Definition One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. Heat of reaction, the amount of heat that must be added or removed during a. Heat Reaction Definition.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Heat Reaction Definition The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. The heat of reaction (also known and enthalpy of reaction) is the. Heat Reaction Definition.
From ar.inspiredpencil.com
Heat Of Reaction Chart Heat Reaction Definition Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. One technique we can use to measure. Heat Reaction Definition.
From chemtogo.weebly.com
Heat in Reactions Chemistry To Go! Heat Reaction Definition One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. The heat of reaction which is also known as reaction enthalpy that is the difference. Heat Reaction Definition.
From www.slideserve.com
PPT Heat in Chemical Reactions PowerPoint Presentation, free download Heat Reaction Definition When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. Calorimetry is used to measure. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat of reaction, the amount of heat that must be. Heat Reaction Definition.
From spmchemistry.blog.onlinetuition.com.my
Heat of Reaction SPM Chemistry Heat Reaction Definition One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction (also known and enthalpy of reaction). Heat Reaction Definition.
From chemistrytalk.org
Heat of Reaction ChemTalk Heat Reaction Definition Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. The heat of reaction (also known and. Heat Reaction Definition.
From www.slideserve.com
PPT Enthalpy An introduction to Chemical Thermodynamics PowerPoint Heat Reaction Definition The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction which is also. Heat Reaction Definition.
From sciencenotes.org
Thermite Reaction Chemistry Demonstration Heat Reaction Definition Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. The heat of reaction which is also known as. Heat Reaction Definition.
From sciencenotes.org
Endothermic Reactions Definition and Examples Heat Reaction Definition The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. Calorimetry is used to measure. When we. Heat Reaction Definition.
From www.chemistrylearner.com
Heat (Enthalpy) of Solution Definition, Formula, & Problems Heat Reaction Definition The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction which is also. Heat Reaction Definition.
From www.worldatlas.com
What Are The Properties Of Matter? WorldAtlas Heat Reaction Definition Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. When we study energy changes in chemical. Heat Reaction Definition.
From mungfali.com
Standard Heat Formation Chart Heat Reaction Definition One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (δh rxn), the change in. Heat of reaction, the amount of heat that must be added or removed during a. Heat Reaction Definition.
From www.chemistrylearner.com
Heat (Enthalpy) of Neutralization Definition and Formula Heat Reaction Definition Heat of reaction, the amount of heat that must be added or removed during a chemical reaction in order to keep all of the substances present at the. The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. The heat of reaction (also known and. Heat Reaction Definition.
From www.youtube.com
Heats of Reaction YouTube Heat Reaction Definition The heat of reaction (also known and enthalpy of reaction) is the change in the enthalpy of a chemical reaction that occurs at a constant. The heat of reaction which is also known as reaction enthalpy that is the difference in the enthalpy of a specific chemical reaction that is. Heat of reaction, the amount of heat that must be. Heat Reaction Definition.